Henri Becquerel's Atomic Theory Henri Becquerel was born into a science family Becquerel did close work with potassium uranyl sulfate, which he exposed to sunlight and placed on photographic plates wrapped in black paper When it was developed, the plates revealed an image of uranium crystals Later experiments;When Henri Becquerel investigated the newly discovered Xrays in 16, it led to studies of how uranium salts are affected by light By accident, he discovered that uranium salts spontaneously emit a penetrating radiation that can be registered on a photographic plateHenri Becquerel made an important contribution to our understanding of atomic theory when he discovered the existence of radioactivity In this lesson, learn about him and his amazing discovery

Main Menu Great Pyramids 2900 e Atomic Theory Founders Of Ppt Video Online Download
Henri becquerel atomic model
Henri becquerel atomic model-Henri Becquerel Atomic Theory from Chapter 14 / Lesson 18 92K Henri Becquerel was a French scientist in the late 1800s who developed an atomic theory, and studied radiation, and light1What was Henri Becquerel studying when he accidentally discovered radioactivity?
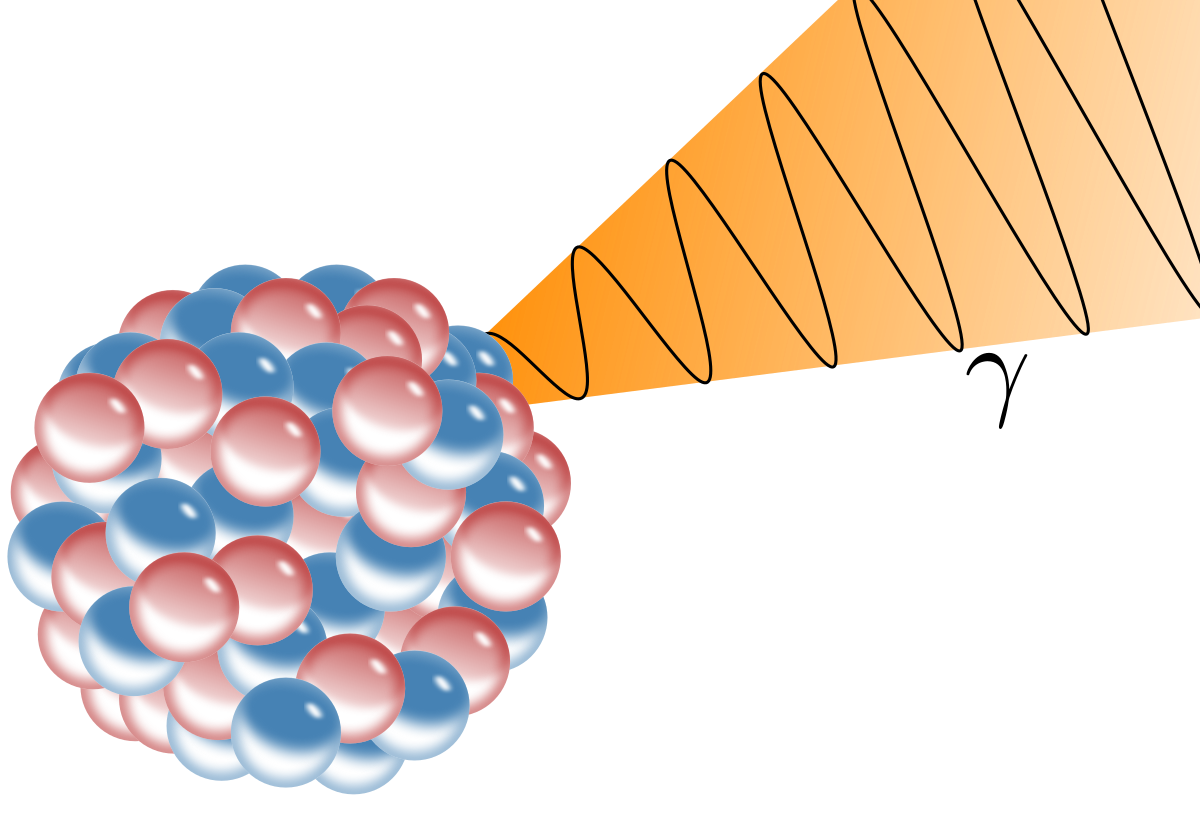



Gamma Ray Wikipedia
Advantages And Disadvantages Of Rutherford's Model Of Atom Atoms are the fundamental building blocks of matter Atom is made up of smaller particles called sub atomic particles Three main subatomic particles are electrons, protons, neutrons The existence of electron in an atom was shown by the scientist JJ Thompson on the basis of detailed In 16, French scientist Antoine Henri Becquerel discovered radioactivity which was an early contribution to atomic theory He discovered this phenomenon while experimenting with uranium and a photographic plate Becquerel began his experiment by exposing a crystal that contained uranium to sunlight After the crystal had soaked up some Radioactivity was discovered in 16 by the French scientist Henri Becquerel while studying the natural phosphorescence of substances Using samples that contained uranium, Becquerel noted that radioactive emissions occurred spontaneously The main types of radioactivity are alpha, beta and gamma emissions Many studies carried out before and after Becquerel
Modern Atomic Theory Radioactive Materials Bequerel's photographic plate In 16, Henri Bequerel was studying the fluorescent properties of uranium salts and placed a piece of the uranium salt on top of a photographic plate wrapped in black paper He discovered, upon development, that the plate was exposed in the shape of the uranium sampleThe Nobel Prize in Physics 1903 was divided, one half awarded to Antoine Henri Becquerel "in recognition of the extraordinary services he has rendered by his discovery of spontaneous radioactivity", the other half jointly to Pierre Curie and Marie Curie, née Sklodowska "in recognition of the extraordinary services they have rendered by their joint researches on the radiation A model of an atom shows eight electrons in rings that represent different energy levels How many electrons are in each energy science Cobalt has a mass number of 59 and an atomic number of 27 A student wants to create a model of a cobalt atom Which statement about the model is correct?
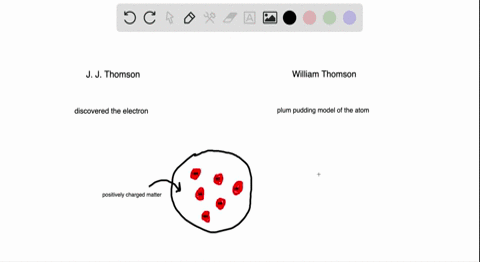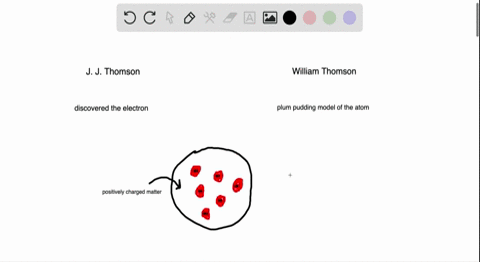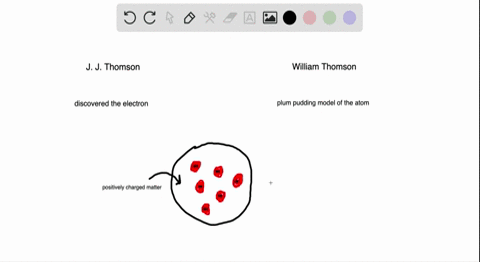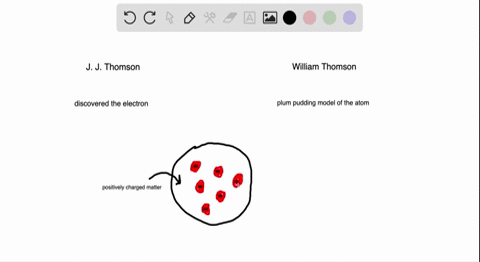
Atomic Theory Review Sheet plum pudding model Henri Becquerel 16 Marie Curie 1905 received Nobel prize (shared with her husband Pierre Curie and Henri Bequerel) for her work in studying radiation Name the three kinds of radiation and describe each typeHenri Becquerel Discoverer of radioactivity Career Henri followed in the footsteps of his ancestors chemistry and physics through his university years at the École Polytechnic Henri joined the government department of PontsetChaussées in 1874, quickly rising through the ranksAlthough Becquerel did not initially comprehend what he was observing, his landmark discovery of radioactivity paved the way for a new understanding of the atom and atomic structure On , Becquerel attended a meeting of the French Academy of Science and presented a short paper (one of the quickest methods in France at that time




Atom Theory By Mykah Stone




Henri Becquerel Atomic Theory Video Lesson Transcript Study Com
AntoineHenri Becquerel was born in Paris on 15 December 1852, the son of the French physicist Alexandre Edmond Becquerel who was known for his work on luminescence and phosphorescence The young Becquerel studied science at the École Polytechnique in Paris where he too became interested in aspects of light absorption, eventually occupying theHenri Becquerel was born into a family of scientists With influences from his father and grandfather, Bequerel worked with properties of the atom, such as magnetism and radioactivity His biggest achievements were in the field of radioactivity In his earlier works, Bequerel worked with light and the absorption of light by crystalsHenri Becquerel Discovered that some chemicals spontaneously decompose and give off penetrating rays while working with Xrays and photography paper 17 JJ Thomson Discovers the electron and used cathode ray tubes to determine the charge to mass ratio of an electron to be 1759 x 10 8 Coulombs/gram JJ Thomson




Chemestry 11 Lessons April 14 The Atomic Theory



Atomic Structure Chemistry The Greeks History Of The
Henri Becquerel Biographical A ntoine Henri Becquerel was born in Paris on , a member of a distinguished family of scholars and scientists His father, Alexander Edmond Becquerel, was a Professor of Applied Physics and had done research on solar radiation and on phosphorescence, while his grandfather, Antoine César, had been a Fellow of the Royal SocietyThe mark of a great scientist is making grand discoveries almost unintentionally While studying fluorescence, Henri Becquerel discovered natural radioactiviAntoine Henri Becquerel Becquerel was born in in Paris France and Died Most of his family members had studied in professional physics He made a pioneering discovery in phosphorescence, and later earned the Nobel Prize in physics



Discovering Particles Into The Atom




Antoine Henri Becquerel His Part Of The Atomic Theory By Grecia Emberger
Henri Becquerel atomic model Wiki User ∙ See Answer Best Answer Copy i need somebody smart to answer this, its for my chemistry project!!!!!Antoine Henri Becquerel (/ ˌ b ɛ k ə ˈ r ɛ l /;Atom atom Discovery of radioactivity Like Thomson's discovery of the electron, the discovery of radioactivity in uranium by French physicist Henri Becquerel in 16 forced scientists to radically change their ideas about atomic structure Radioactivity demonstrated that the atom was neither indivisible nor immutable Instead of serving merely as an inert matrix for electrons, the atom




Atomic Theory Timeline By Skye Clark And Megan Barry



Atomic Theory
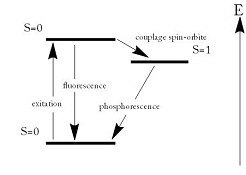
Henri Becquerel, in full AntoineHenri Becquerel, (born , Paris, France—died , Le Croisic), French physicist who discovered radioactivity through his investigations of uranium and other substances In 1903 he shared the Nobel Prize for Physics with Pierre and Marie Curie He was a member of a scientific family extending through severalThe atomic mass for sulphur is 32 amu or (Atomic Mass Unit is the international system of units) The melting point of sulphur is 1128 °C and the boiling point of Sulphur is 4446 °C Sulphur has an equal number of proton and neutron due to its atomic mass subtracted by the proton/atomic number SulphurRUTHERFORD'S EXPERIMENT CENTRAL NUCLEUS The earlier "plum pudding" theory of atomic structure was shattered and a new theory of central nucleus was put forward FROM CATHODE RAYS TO XRAYS XRAYS CATHODE RAYS & XRAYS HENRI BECQUEREL'S EXPERIMENT Becquerel's Conclusion JJ THOMSON'S 'PLUM PUDDING MODEL' OF AN ATOM ALPHA AND




Need To Know Historical Outline Of Radioactivity Work Of Becquerel Discovery Of Radiation From Uranium Salts Marie And Pierre Curie Discovery Of Polonium Ppt Download



Antoine Henri Becquerel Atomic Model Clip Art Library
15 December 1852 – 25 August 1908) was a French engineer, physicist, Nobel laureate, and the first person to discover evidence of radioactivityFor work in this field he, along with Marie SkłodowskaCurie (Marie Curie) and Pierre Curie, received the 1903 Nobel Prize in PhysicsThe SI unit for radioactivity, the becquerel (Bq), is namedAtoms are tiny particles Atoms cannot be divided into Science I'm kinda in a rush but take your time!Thx!The existence of subatomic particles is further confirmed with the discovery of radioactivity by Henri Becquerel The History Of The Atomic Theory and Michael Farraday Essay Sample A Series of Discoveries A consummate experimentalist, Rutherford (1871–1937) was responsible for a remarkable series of discoveries in the fields of radioactivity



Science Civilization And Society




13 Atomic History Development Ideas Atomic Theory Atom Famous Scientist
He suggested that these particles moved within the positively charged atomic body, explaining the atom's neutral charge 2 After further refining his theory, he received the 1906 Nobel Prize in Physics In 18 Gerhard Schmidt of Germany began to investigate the question of whether or not other elements naturally emitted Becquerel raysHenri Bequerel was born on , he wa s a physicist with along side Marie Curie discovered radioactivity And in 1903 they won the Nobel Prize He eventually died Marie Curie was born in France She was famous for pioneering the development of radioactivity, she was the first woman to win the What did Henri Becquerel discover about the atomic theory?




Physicist Page Henri Becquerel The Discovery Of Radioactivity On 12 June 1901 The French Physicist Henri Becquerel Was Doing A Demonstration At The Academy Of Sciences Of Paris For A New



The Atom
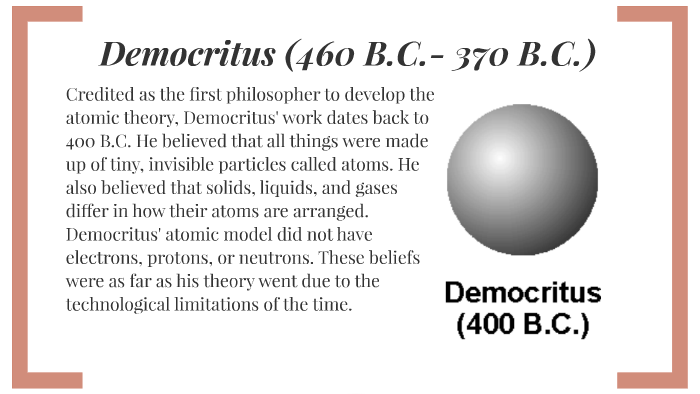
His reward Even though he had discovered radioactivity, he hadn't pursued into it Instead Marie and Pierre Curie did most of the studies on radioactivity There fore the three had split the Nobel Prize of Physics in 1903 "Henri Becquerel Biography" Nobelprizeorg 17 JanHenri Becquerel 16 •worked with properties of the atom, such as magnetism and radioactivityfound that some atoms have natural radioactivity •His discovery of radioactivity allowed later scientists to perfect the atomic model Democritus 400 BCANTOINE HENRI BECQUEREL In 16 Antoine Henri Becquerel had a piece of mineral which contained Uranium, it produced it's image on a photographic plate in the absence of light What he discovered was radioactivity Radioactivity was a big part in the Atomic Theory because it proved that there are smaller particles in an atom that makes it up



Solved What Discoveries Were Made By J J Thomson Henri Becquerel And Lord Rutherford How Did Dalton S Model Of The Atom Have To Be Modified To Account For These Discoveries Picture Cant Copy



Henri Becquerel And The Discovery Of Radioactivity Ans Nuclear Newswire
Major Accomplishments Henri Becquerel's research was one of the most important of his time He was awarded the Nobel Prize, the Rumford Medal, the Helmholtz Medal, and the Barnard Medal for his great achievement Throughout his life he conducted tests involving Uranium and other substances It was with these experiments that he discovered , was the birthday of French physicist Antoine Henri Becquerel, who discovered a completely unknown property of matter in March 16 Becquerel Some might say Becquerel's discovery of "radioactivity" was a lucky accidentbut as the Roman philosopher Seneca wrote in the 1st century, "Luck is what happens when preparation meetsBecquerel put the crystals in



Marie Curie




Henri Becquerel Biography Facts And Pictures
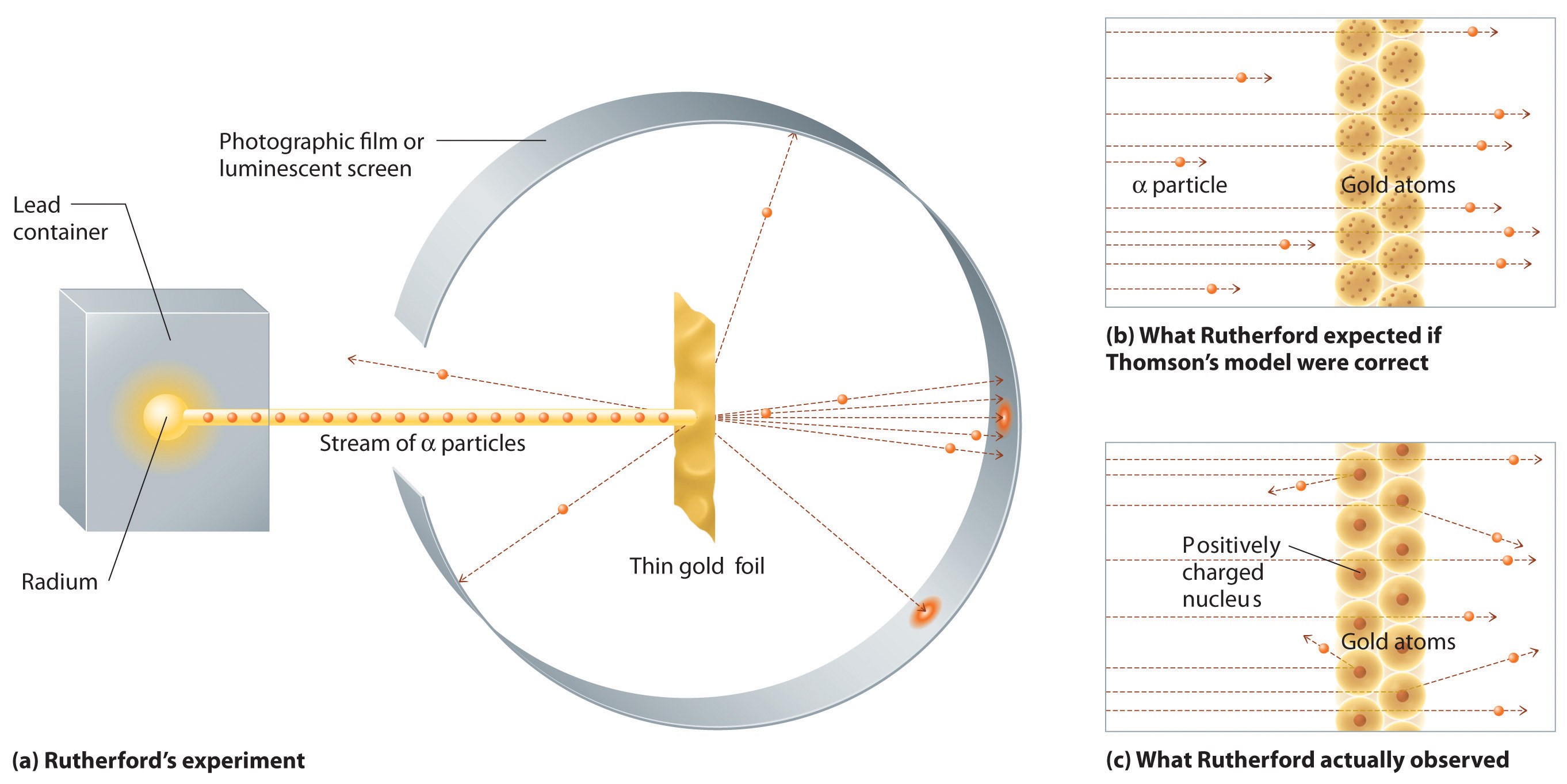
Which of the following parts of Dalton's atomic model was disproved by the discovery of the electron?Henri Becquerel 16 Discovered radioactivity Radiation produced spontaneously by unstable atomic nucleus Thomson's Atom 17 Discovered electrons Plum Pudding model (for us Americans it looks like a chocolate chip cookie model) Electrons charge charge "pudding" Rutherford's Atom 1909 discovered dense positively charged nucleusThe discovery of radioactivity in uranium by French physicist Henri Becquerel in 16 forced scientists to radically change their ideas about atomic structure Thomson's Experiment Thomson's experiments with cathoderay tubes showed that all atoms contain tiny negatively charged subatomic particles or electrons




Radioactivity How Was Radioactivity Discovered In 16 The Scientist Henri Becquerel Left A Piece Of Uranium Rock On A Photographic Plate In His Ppt Download




History Of An Atom Timeline Timetoast Timelines
Henri Becquerel Antoine Henri Becquerel () was a French physicist and winner of the 1903 Nobel Prize in Physics Becquerel was born in Paris, France on He was the son of a professor of applied physics, Alexander Becquerel He began his studies in 1872 at École Polytechnique just south of ParisProposed the Atomic Theory (1) matter consists of tiny particles called atoms, (2) atoms cannot be created, destroyed, subdivided or converted from one type to another by chemical means, (3) atoms of a particular element all have the same properties Henri Becquerel (16) Discovered some chemicals decompose and give off penetrating rays



Untitled Document




Atomic Theory Timeline Project



Modern Theory Of Atomic Structure By Adam Dirosa




Solved What Discoveries Were Made By J J Thomson Henri Becquerel And Lord Rutherford How Did Dalton S Model Of The Atom Have To Be Modified To Account For These Discoveries Picture Cant Copy



Periodic Table




Atomic Theory By Courtney Carroll



Electronic Structure Of Atoms Dalton S Atomic Model




Development Of Atomic Structure Atom Models Bohr Chemistry En Nuclear Physics Plum Pudding Pt Science Scientists Set Glogster Edu Interactive Multimedia Posters




International Atomic Energy Agency Iaea French Physicist Henri Becquerel Discovered Radioactivity Onthisday In 16 Like So Many Other Important Scientific Discoveries This One Was Also Accidental 26 February 16 Was An




Atom Discovery Of Radioactivity Britannica
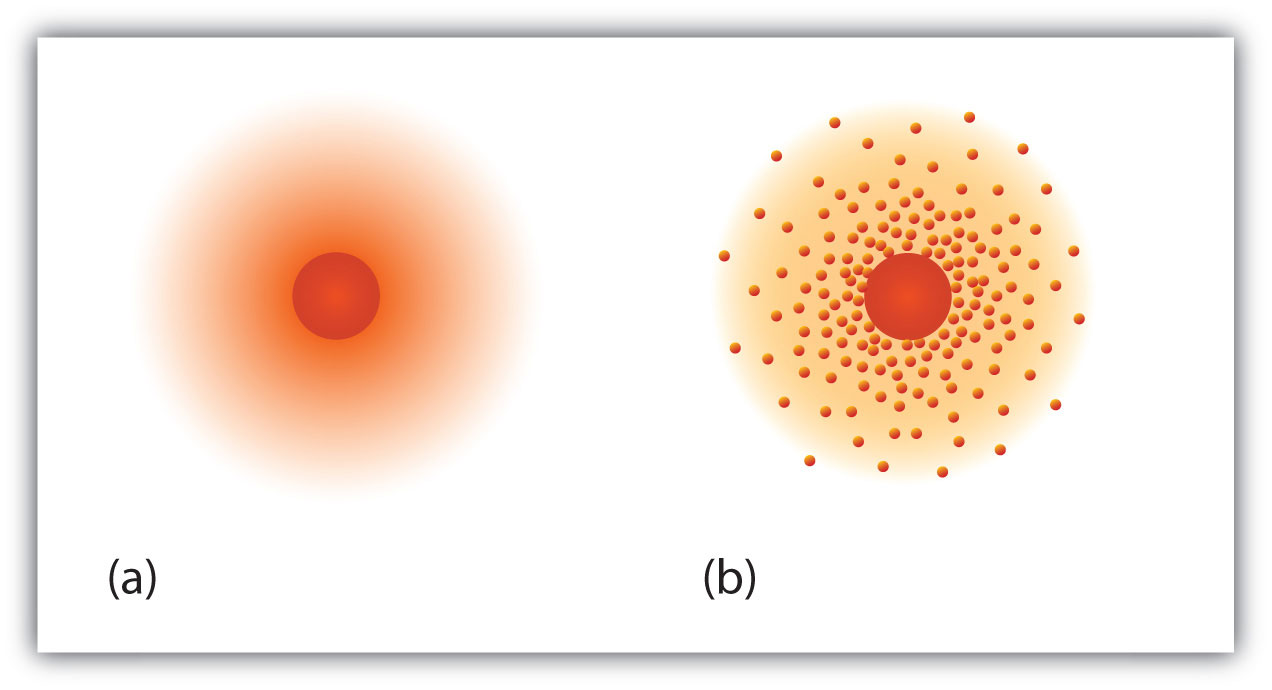



Ch104 Chapter 2 Atoms And The Periodic Table Chemistry




Chapter 8 Nuclei By Qin Qin Fei And Wendy Martinez Ernest Rutherford Ernest Rutherford Was Born In 1871 In The South Island Of New Zealand He Was The Fourth Of Twelve Children Of James And Martha Rutherford Who Had Earlier Emigrated From The United




Nuclear Chemistry Wikipedia
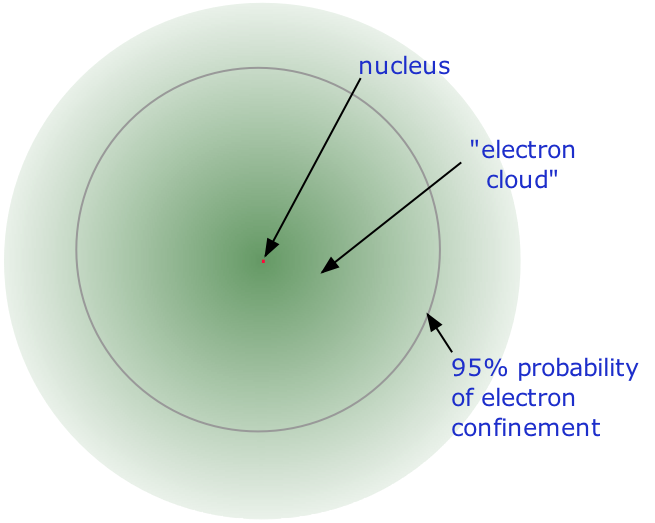



2 1 Atoms Their Composition And Structure Chemistry Libretexts
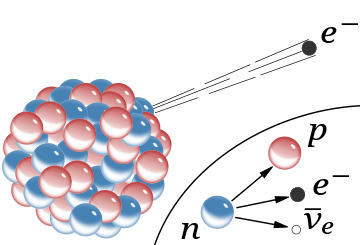



Discovery Of The Neutron Wikipedia




Main Menu Great Pyramids 2900 e Atomic Theory Founders Of Ppt Video Online Download




Henri Becquerel Atomic Heritage Foundation
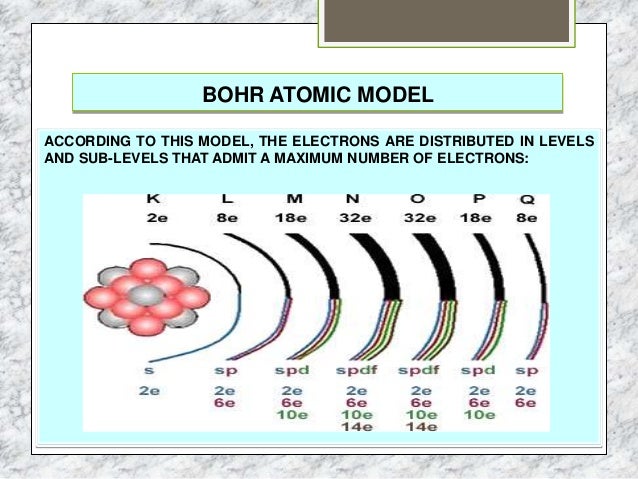



Theme 2 The Atom



Historical Atomic Theory Developments In Atomic Theory From




16 Atomic Theory Ipc Princeton
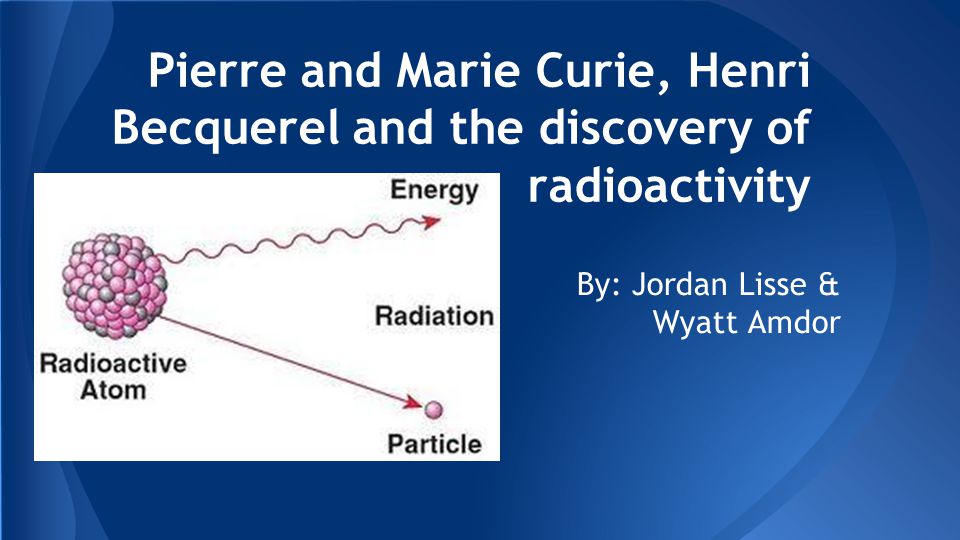



Pierre And Marie Curie Henri Becquerel And The Discovery Of Radioactivity By Jordan Lisse Wyatt Amdor Ppt Download




Lesson 5 Atomic Theory Anything In Black Letters



Best Teachers Pages Library Projects Early Models Of The Atom Scavenger Hunt Chem 11 Ms Hamilton




Atom Discovery Of Radioactivity Britannica




Ppt Atom Theory Development Timeline Powerpoint Presentation Free Download Id
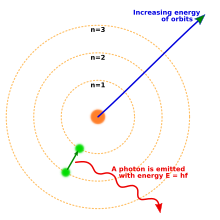



Electron Wikipedia



Henri Becquerel And Marie Curie
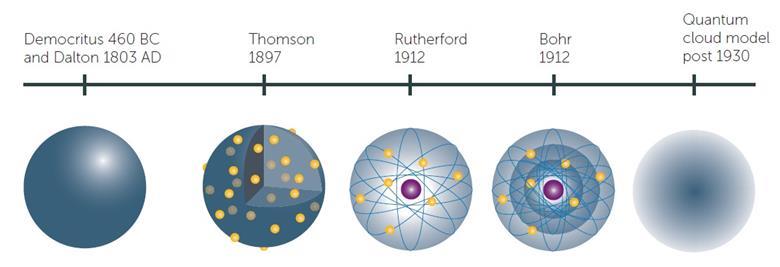



Atomic Theory By Rubykrasnow On Emaze



Henri Becquerel By Ana Jaramillo Infographic
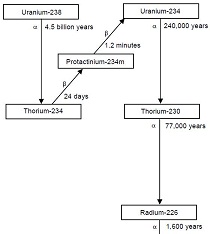



Henri Becquerel And The Discovery Of Radioactivity Ans Nuclear Newswire




Science Concepts And Questions K To 12 The Structure Of The Atom



Atomic Theories Timeline




Atomic Theory Timeline Do The Evolution




Radioactive Decay Wikipedia




1 Atomic Physics 2 In 16 Henri Becquerel Discovered That Certain Uranium Compounds Would Fog Photographic Plates As If Exposed To Light He Discovered Ppt Download




Atom Discovery Of Radioactivity Britannica




Alpha Particle Scattering Experiment Ck 12 Foundation



The Discovery Of Atomic Structure



Henri Becquerel And Marie Curie




Basic Nuclear Science Information




Thomson Atomic Model Basis Limitations And Drawbacks




Lecture 3 Fall Chapter 2 Atoms Molecules And Ions Ppt Download




James Chadwick S Atomic Theory And Its Lasting Impact Explained Science Struck




Physical Science Quantum Mechanics Britannica




Atomic History And Theory Flashcards Quizlet




Radioactivity The Unstable Nucleus Radioactivity Is The Spontaneous Breaking Up Of An Unstable Nucleus With The Emission Of Radiation Ppt Download
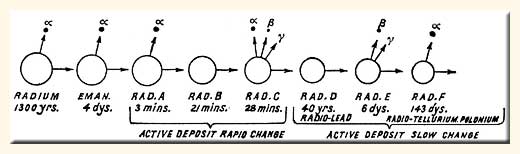



Marie Curie The Unstable Nucleus And Its Uses




Ppt John Daltons Atomic Theory Powerpoint Presentation Free Download Id



The Scientific Odyssey The Atom




Atom Discovery Of Radioactivity Britannica
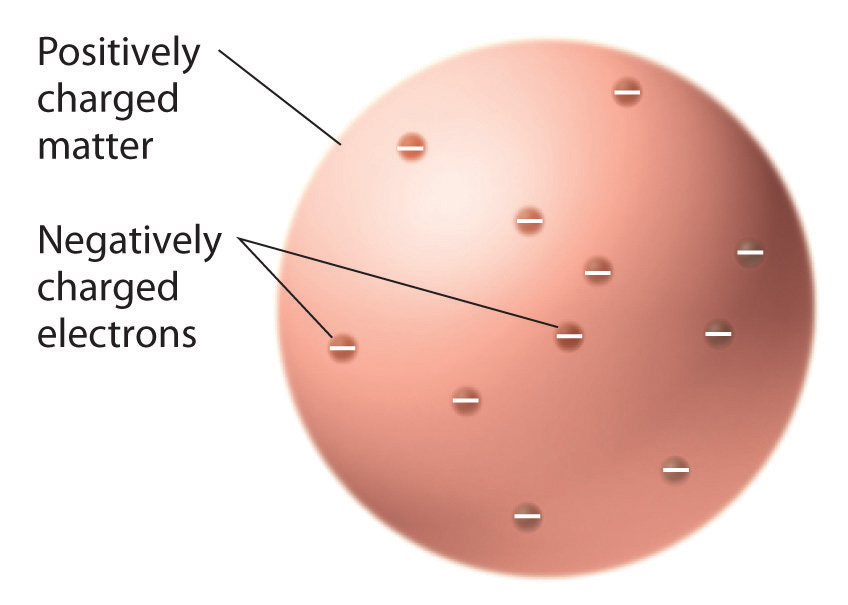



2 2 The Discovery Of Atomic Structure Chemistry Libretexts




Gamma Ray Wikipedia



The Discovery Of Atomic Structure
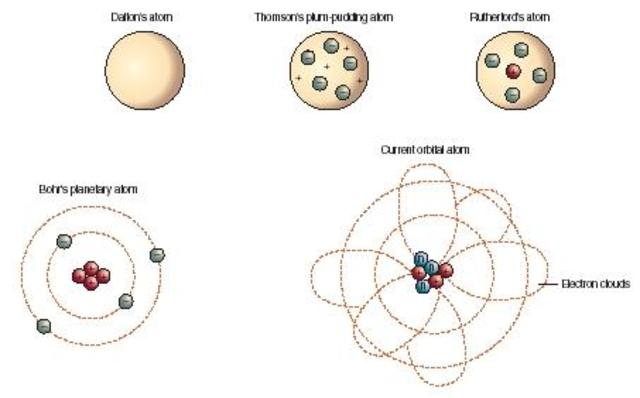



History Of The Atom Chase Vickery Timeline Timetoast Timelines



Evolution Of Atomic Theory Course Hero



Unf Edu




March 1 16 All Aglow Wired
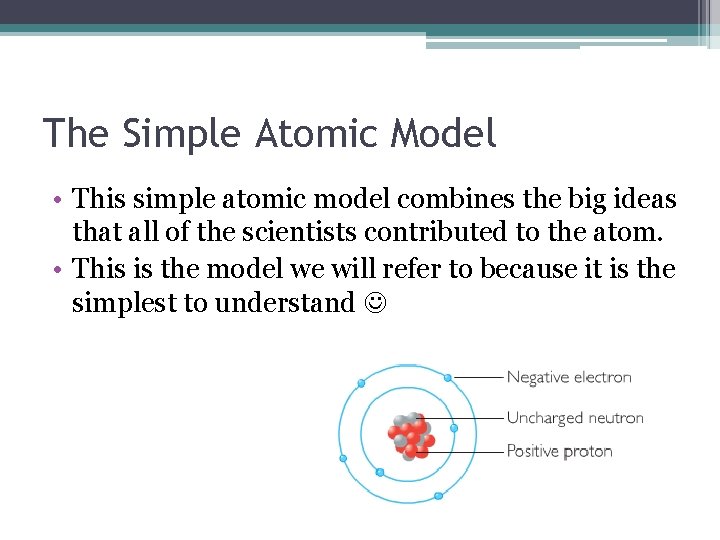



Chapter 3 Atoms The Building Blocks Of Matter



Atomic Structure Presentation Chemistry
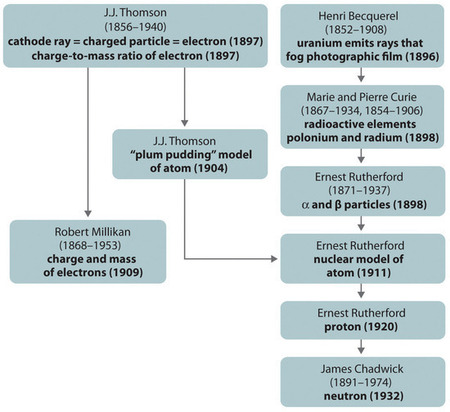



2 2 The Discovery Of Atomic Structure Chemistry Libretexts




Plum Pudding Model Of An Atom Plum Pudding Model Atom Plum Pudding



Ppt The Development Of Atomic Theory Powerpoint Presentation Free Download Id



Selected Classic Papers From The History Of Chemistry



Development Of The Atomic Theory




Atomic Model Timeline Preceden
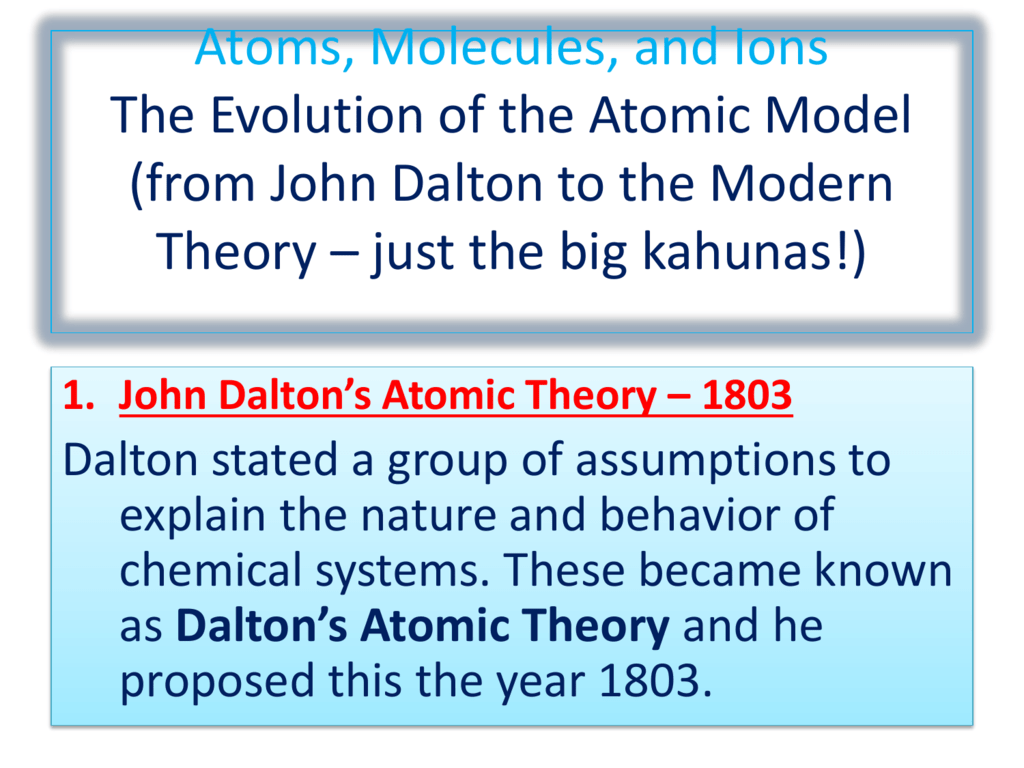



Atoms Molecules And Ions The Evolution Of The Atomic Model From




1a History Of The Atom 2



1
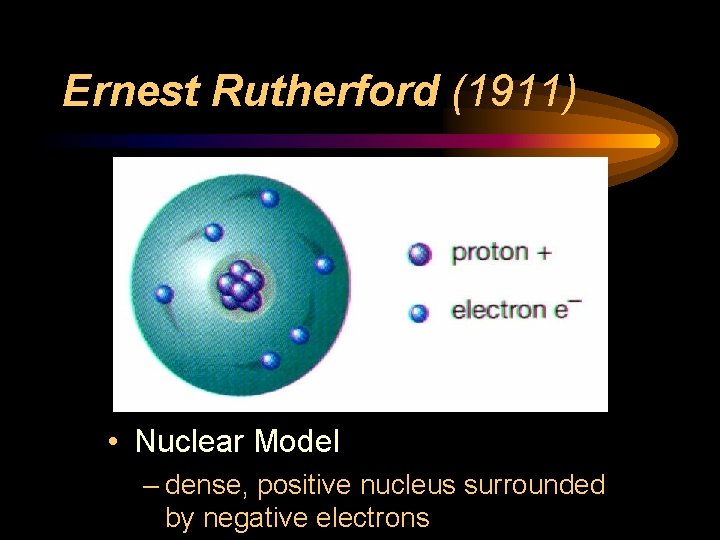



Atomic Structure Timeline Use The Following Information To




Atomic Structure An Overview Sciencedirect Topics




Atomic Theory And Atomic Structure Help And Review Videos Lessons Study Com



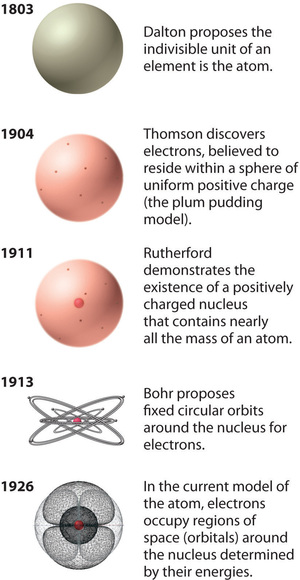
Moseley Fezeks



Atomic Theory Civ5 Civilization Wiki Fandom




Chpt 4 Atomic Theory



The Discovery Of Atomic Structure



Real Atom




Radioactivity Discovering The Nucleus The Proton And The Neutron How Atoms Work Howstuffworks




Atom Discovery Of Radioactivity Britannica




Atom Theory By Mykah Stone




International Atomic Energy Agency Iaea Happy Birthday To Henri Becquerel This French Physicist Shared The Nobel Prize In Physics With Marie Curie For Their Discovery Of Radioactivity In 16 Becquerel And




What Is An Atomic Model Timeline Of Atomic Models



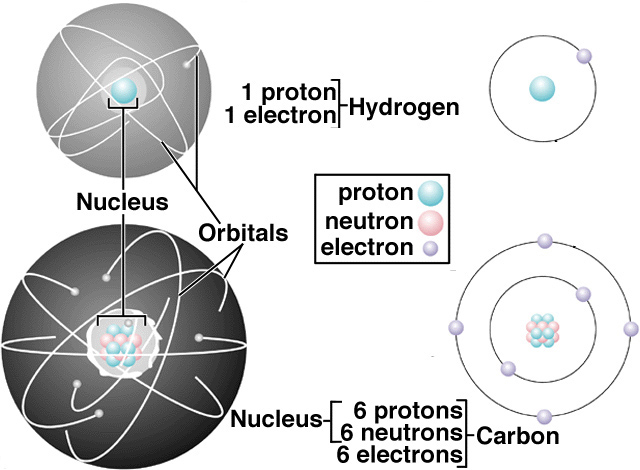
2 2 The Discovery Of Atomic Structure Chemistry Libretexts




Yoair Blog The World S Anthropology Blog Publication



Atomic Structure Timeline Presentation Chemistry



No comments:
Post a Comment